This article is a part of Climate Action at Penn State, a blog highlighting climate solutions, research, and other efforts at Penn State.
I was recently named a fellow for the Energy 2100 initiative. Energy 2100 is a project that aims to drive additional research, innovation, and education in technologies that could be used for providing energy, in the form of transportable fuels and electricity, in an environmentally sound manner in order to use our planet’s resources in a sustainable manner.
I want to bring researchers who study different components of electrochemistry together from across all of Penn State’s campuses, and Energy 2100 is one way to help me do that. Currently, there is an opportunity to bring more of these researchers together to make even better progress than our current arrangement allows.
Electrochemistry is a foundation for our transition from fossil fuels to carbon-neutral life. Electrochemistry is the basic science underlying batteries, solar cells, fuel cells, and the conversion of CO2 into useful products. It is necessary for us to seriously achieve a sustainable energy future.
In our lab, we are working on solutions to major environmental challenges by using electrochemical principles to solve environmental problems. We are adapting the principles used in batteries and fuel cells to discover new ways to purify water, produce renewable electricity, increase the efficiencies of industrial practices, and capture carbon dioxide from the air.
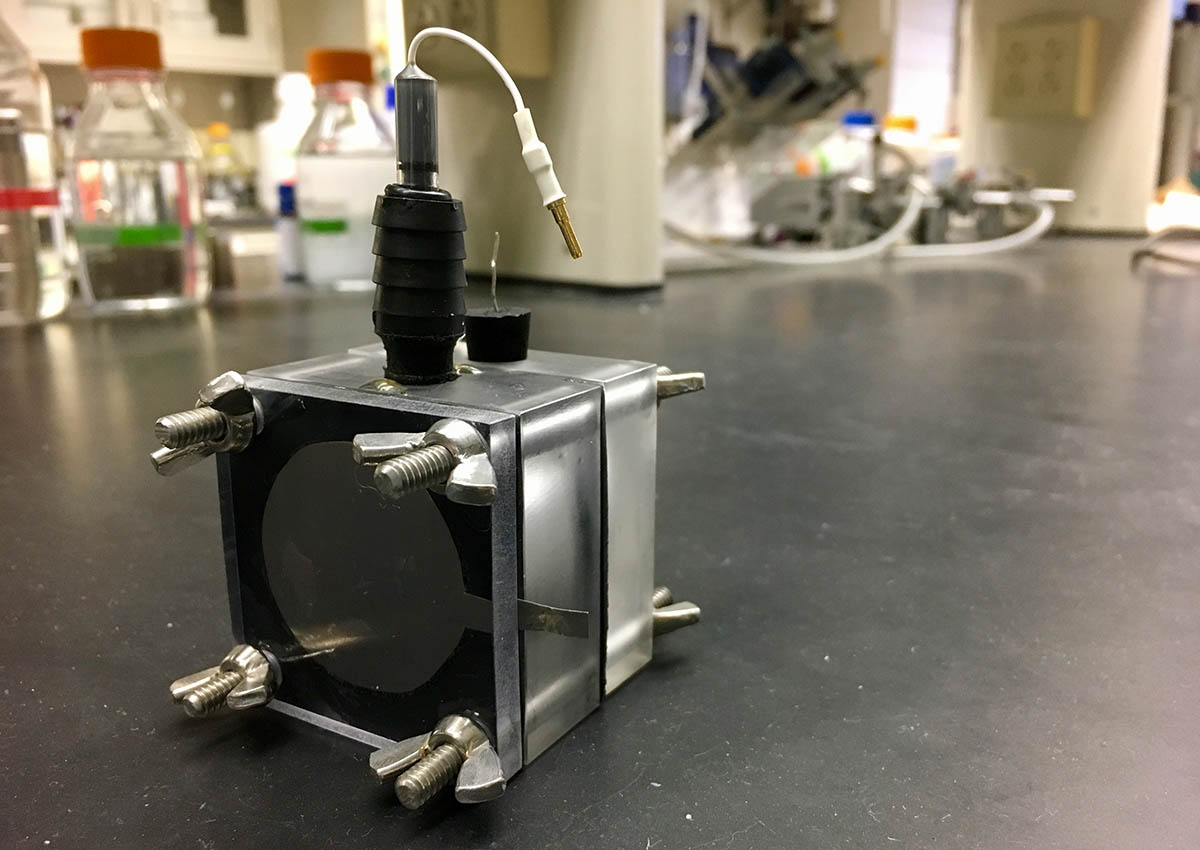
Pictured above is a three-electrode electrochemical cell used to study the electrochemical properties of intercalating materials, or materials altered by inserting layers of other molecules between layers of original material. The intercalating materials can be used to electrochemically harvest renewable energy from salinity gradients and desalinate water.
My goal is to meet with faculty studying electrochemistry and identify how we can strategically work together to better address climate change issues and improve awareness of research and education being done at Penn State. Climate change is transforming this world in ways that can be devastating. It is a time-sensitive issue. The longer we wait, the more difficult it will be to deal with.


